Regulatory Submissions Tracking Tool
A centralized system built to help AS&A manage and monitor all regulatory filings submitted on behalf of their clients, replacing scattered spreadsheets and manual updates with a unified workflow and automated follow-ups.
From the partner
The Regulatory Submissions Tracking Tool was developed for AS&A (Abel Santos & Asoc.) as an internal platform to manage the complex regulatory submissions process for their pharmaceutical and laboratory clients.
Built with Airtable as the database and Softr as the interface, the tool consolidates all submissions by client, laboratory, and regulatory body, providing real-time visibility into filing status, workload, and deadlines.
Key capabilities include:
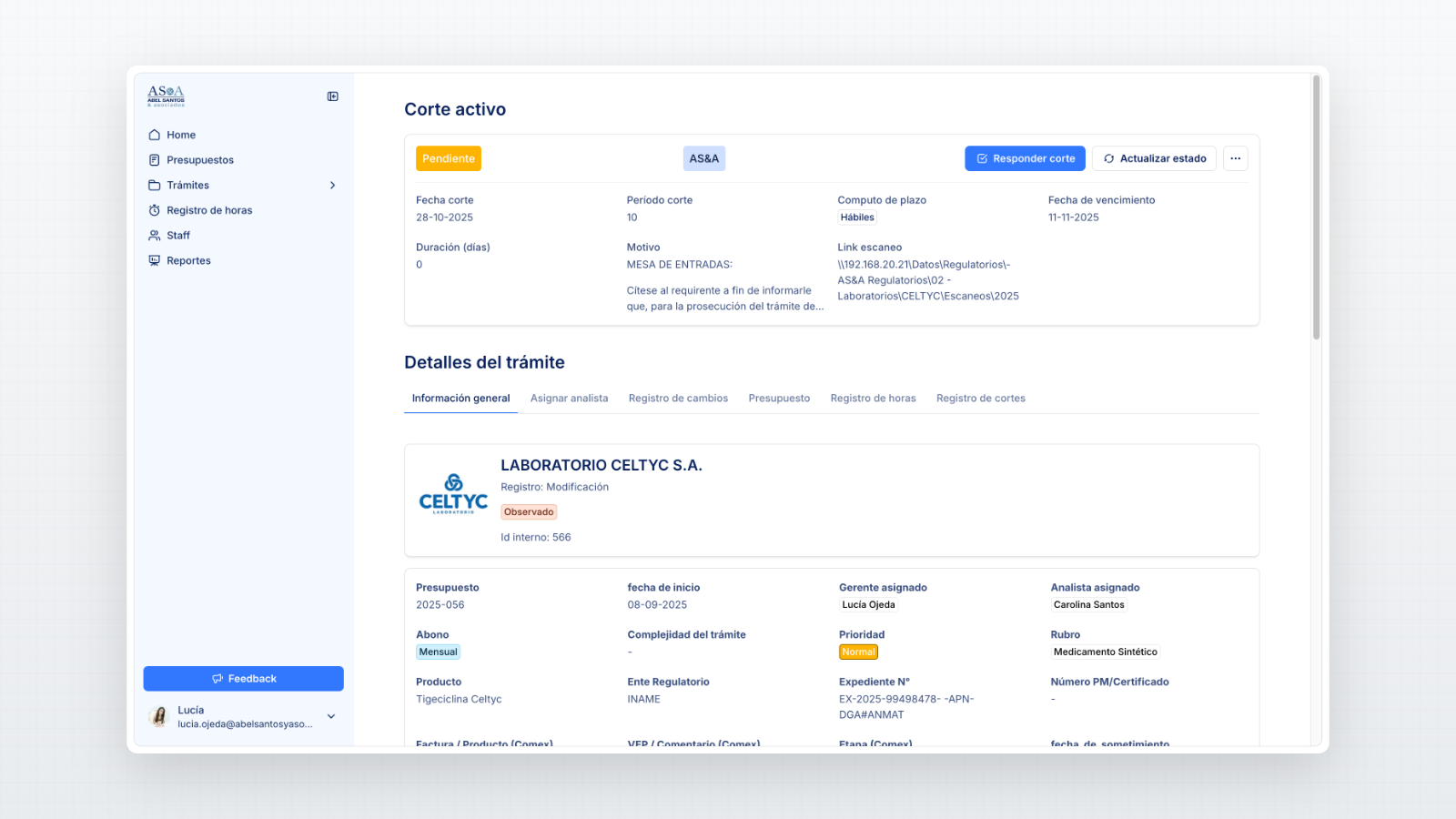
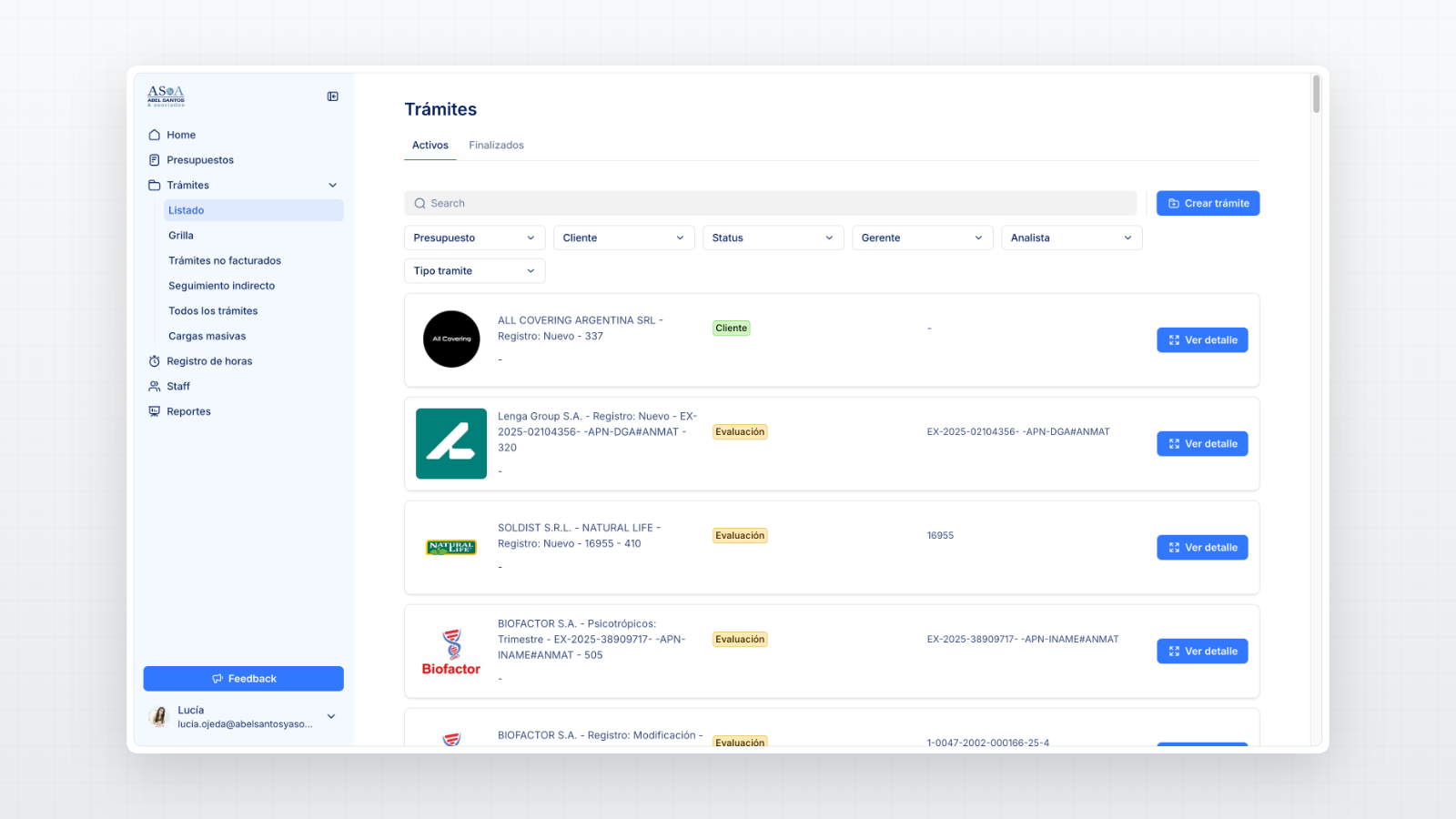
A centralized database to organize every regulatory filing from submission to resolution.
Automated reminders for agency follow-ups and client communications.
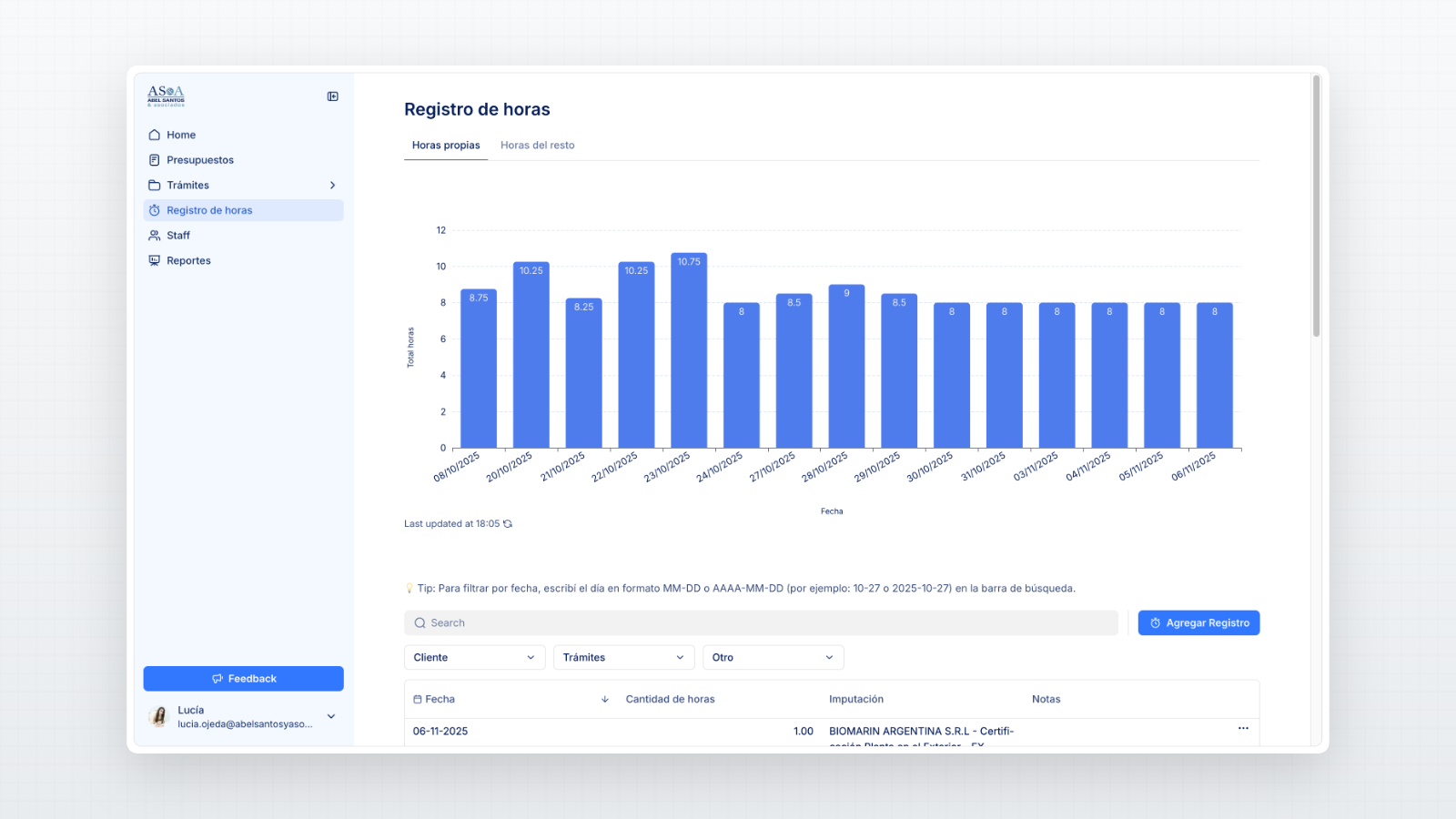
Dashboards to monitor workload distribution, pending approvals, and performance.
Document storage linking each filing to its official correspondence and responses.
Custom Airtable reports offering management insight into bottlenecks and client portfolio activity.
By integrating these processes into one platform, the solution improved operational efficiency, reduced manual errors, and provided laboratories and clients with faster and clearer updates on the progress of their regulatory submissions.